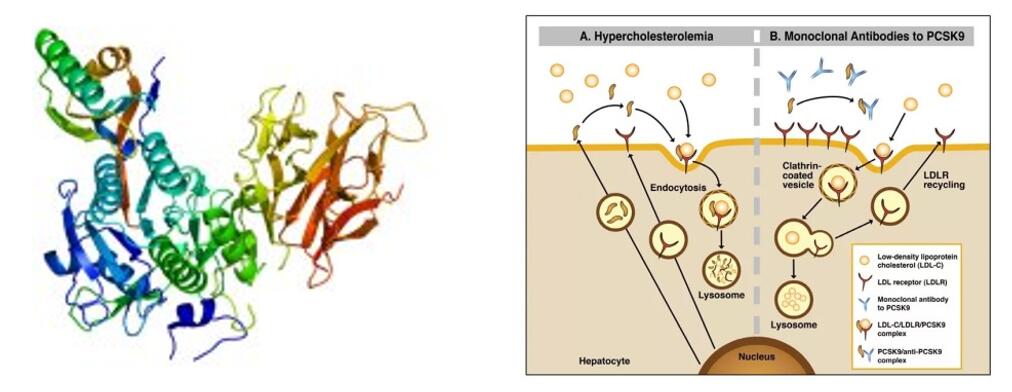
Recently, Chinese State Food and Drug Administration (SFDA) officially accepted the marketing application of tafolecimab (PCSK-9 Monoclonal antibody which is made by INNOVENT BIOLOGICS,INC), INC for the treatment of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia and non-familial hypercholesterolemia) and mixed dyslipidemia. This is the first self-produced PCSK-9 inhibitor to apply for marketing in China.
Tafolecimab is an innovative biologic drug independently developed by INNOVENT BIOLOGICS, INC. IgG2 human monoclonal antibody specifically binds PCSK-9 to increase LDLR levels by reducing PCSK-9-mediated endocytosis, thereby increasing LDL-C elimination and lowering LDL-C levels.
In recent years, the prevalence of dyslipidemia has increased significantly in China. The prevalence of dyslipidemia and hypercholesterolemia in adults is as high as 40.4% and 26.3% respectively. According to the 2020 report on Cardiovascular Health and Diseases in China, the treatment and control rate of dyslipidemia in adults are still at a low level, and the LDL-C compliance rate of dyslipidemia patients is even less satisfactory.
Previously, statins were the main treatment for hypercholesterolemia in China, but many patients still failed to achieve the treatment target of LDL-C reduction after treatment. The marketing of PCSK-9 has brought better efficacy to patients.
Submission of tafolecimab from INNOVENT BIOLOGICS, INC is based on the results of three clinical trials registered in a democratic stage, It has a good overall safety profile, similar to the safety characteristics of marketed products, and has achieved long intervals (every 6 weeks) of administration. The results of the CREDIT-2 study were accepted by the 2022 Annual Meeting of the American College of Cardiology (ACC) as an abstract and published online.
If the application is approved, it will break the deadlock of impoeted PCSK-9, China will become the fourth country to have PCSK-9 after the United States (Amgen), France (Sanofi) and Switzerland (Novartis).
Post time: Jul-04-2022