Medical device registration certificate of the People’s Republic of China
Medical device registration refers to the process of systematically evaluating the safety and effectiveness of medical devices to be sold and used in accordance with legal procedures, so as to decide whether to agree to their sale and use. It is divided into Chinese domestic medical device registration and overseas medical device registration. Overseas medical devices, whether class I, class II or class III, should be handled by the Beijing State Food and Drug Administration: domestic class I and class II medical devices should be handled by the local provincial or municipal food and drug administration, and class III medical devices should be handled by the State Food and drug administration. Medical device registration certificate refers to the legal ID card of medical device products.
According to the regulations on the supervision and administration of medical devices, the measures for the supervision and administration of medical device production and the measures for the administration of medical device registration issued by the State Food and drug administration, the medical device products produced and / or sold in China shall meet the corresponding regulatory requirements. These requirements include:
1) The medical device manufacturer obtains the production license;
2) Medical device products have obtained registration certificate.
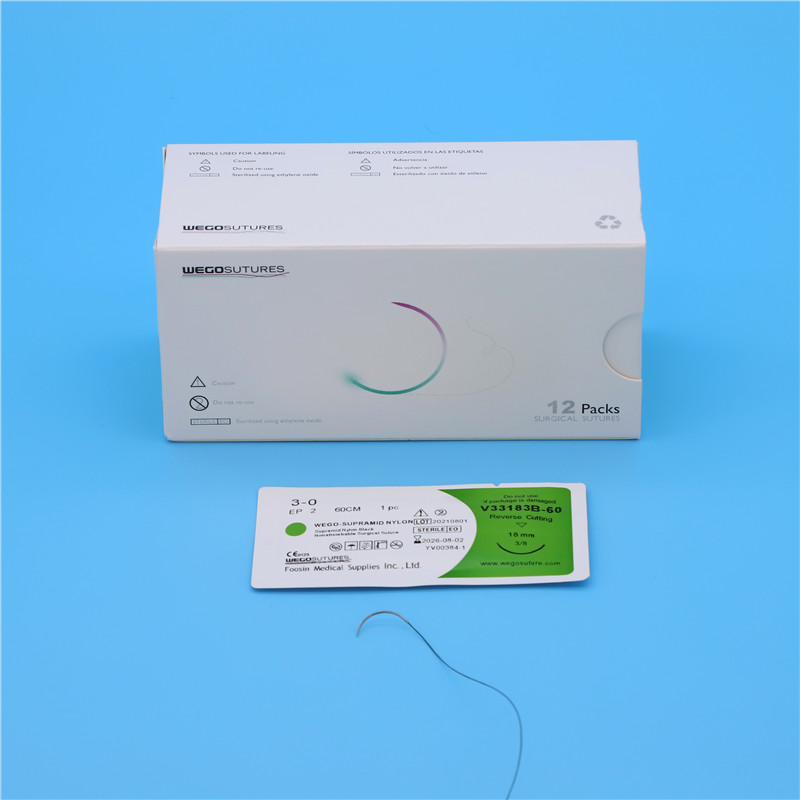
Foosin already obtain Medical Registration in China since 2006 the latest version as following:
Registration Certificate No.: lxzz 20152020252
|
Name of registrant |
Foosin medical materials Co., Ltd |
|
Domicile of registrant |
20, Xingshan Road, Weihai Torch Hi-tech Science Park |
|
Production address |
20, Xingshan Road, Weihai Torch Hi-tech Science Park |
|
Name of agent |
|
|
Agent's domicile |
|
|
Product name |
Non absorbable surgical suture |
|
Model and specification |
See attached annex |
|
Structure and composition |
The product consists of suture needle and non-absorbable surgical suture. |
|
Scope of application |
It is suitable for sewing human tissue. |
|
enclosure |
Product technical requirements: lxzz 20152020252 |
|
Other contents |
|
|
remarks |
Original medical device registration certificate No.: lxzz 20152650252 |
| Approved by: Shandong Provincial Drug Administration |
|
Approval date: March 25, 2020 |
|
Valid until: March 24, 2025 |
|
(seal of approval department) |
Attachment:
| Product name | Nylon | Polypropylene | Polyester | Silk |
| USP | 10-(0#-2#) | 10-(0#-2#) | 8-(0#-2#) | 8-(0#-5#) |
| Suture length | 30cm-299cm | 45cm-299cm | 45cm-299cm | 30cm-299cm |
| Needle diameter × Chord length (0.1mm×mm) |
(1.5-15)×(4.5-55) | (2-15)×(6-55) | (2-15)×(6-55) | (1.5-15)×(6-65) |
| Curve | 1/2, 3/8, 1/4, 5/8 | 1/2, 3/8, 1/4, 5/8 | 1/2, 3/8, 1/4, 5/8 | 0,1/2, 3/8, 1/4, 5/8 |
| Needle type | Round body, cutting, spatula | Round body, cutting, taper cut | Round body, cutting | Round body, cutting, taper cut |
| Needle quantity | 0-8 | 0-8 | 0-8 | 0-16 |